Why in news?
A high-profile potential vaccine for COVID-19 being tested by researchers at Oxford University failed to protect vaccinated monkeys from being infected by the nCoV-2 virus.
On a positive side, the test animals appeared to be protected from pneumonia.
Details
The vaccine candidate: ChAdOx1 nCoV-19 – is a weakened form a common cold virus (adenovirus) that affects chimpanzees but has been neutered to prevent replication in humans.
Virus specific neutralising antibodies were detected in those macaques vaccinated and no such antibodies were seen in those that didn’t get the vaccine.
Taking it to the Human trials
Based on these results 1,110 people are taking part in human trial, half receiving the vaccine and the other half (the control group) receiving a meningitis vaccine.
The dose of the vaccine was half that of what is being used for humans right now.
What are Clinical trials?
Clinical trials or Human Trials are experimental studies on human participants that helps to determine the safety concerns, effectivity and potency of a particular treatment (drugs, vaccines, dietary supplements, dietary choices, medical devices, etc.)
While preclinical research answers basic questions about a drug’s safety, it is not a substitute for studies of ways the drug will interact with the human body – Hence, Clinical Trials are very important in understanding the effects of administering a particular treatment.
Human Clinical Trial Phases
Preclinical Phase (Phase 0)
- Drug and device testing begin with extensive laboratory research which can involve years of experiments in animals and human cells.
- If the initial laboratory research is successful, researches send the data to the Food and Drug Administration (FDA) for approval to continue research and testing in humans.
- Once approved, human testing of experimental drugs and devices can begin and is typically conducted in four phases.
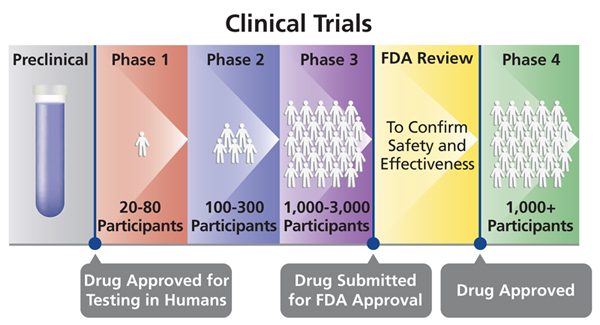
Phase I
- Phase I studies assess the safety of a drug or device. Usually includes a small number of healthy volunteers (20 to 100).
- The study is designed to determine the effects of the drug or device on humans including how it is absorbed, metabolized, and excreted.
- This phase also investigates the side effects that occur as dosage levels are increased.
Phase II
- Phase II studies test the efficacy of a drug or device. Involves up to several hundred patients.
- Most phase II studies are randomized trials where one group of patients receives the experimental drug, while a second “control” group receives a standard treatment or placebo.
- Often these studies are “blinded” which means that neither the patients nor the researchers know who has received the experimental drug.
Phase III
- Phase III studies involve randomized and blind testing in several hundred to several thousand patients. This large-scale testing, which can last several years.
- Once Phase III is complete, a pharmaceutical company can request FDA approval for marketing the drug.
Phase IV
- Phase IV studies, often called Post Marketing Surveillance Trials, are conducted after a drug or device has been approved for consumer sale.
- Phase IV studies can result in a drug or device being taken off the market or restrictions of use could be placed.
-Source: The Hindu




