Context:
According to a new study Emissions of industrially produced chlorocarbon, dichloromethane (CH2Cl2), increased in China from 2011-2019.
Relevance:
GS-III: Environment and Ecology
Dimensions of the Article:
- What is Ozone Layer and what are Ozone Holes?
- Ozone creation and destruction
- Agreements and steps to address the concerns
- About China’s Ozone-destroying greenhouse gas emissions
What is Ozone Layer and what are Ozone Holes?
- Ozone layer, also called ozonosphere, is a region of the upper atmosphere, between roughly 15 and 35 km (9 and 22 miles) above Earth’s surface which contains relatively high concentrations of ozone molecules (O3).
- Approximately 90 percent of the atmosphere’s ozone occurs in the stratosphere, the region extending from 10–18 km (6–11 miles) to approximately 50 km (about 30 miles) above Earth’s surface.
- The ozone layer effectively blocks almost all solar radiation of wavelengths less than 290 nanometres from reaching Earth’s surface, including certain types of ultraviolet (UV) and other forms of radiation that could injure or kill most living things.
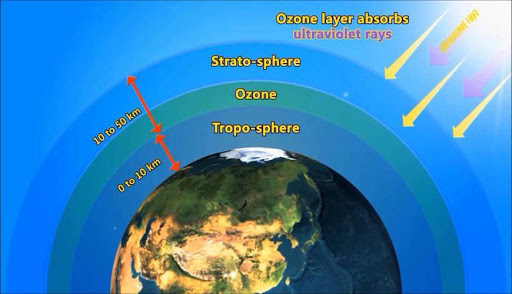
What are Ozone Holes?
- The ‘ozone hole’ is not really a hole — it refers to a region in the stratosphere where the concentration of ozone becomes extremely low in certain months.
- The ‘ozone holes’ most commonly talked about are the depletions over Antarctica, forming each year in the months of September, October and November, due to a set of special meteorological and chemical conditions that arise at the South Pole, and can reach sizes of around 20 to 25 million sq km.
- Such holes are also spotted over the North Pole, but owing to warmer temperatures than the South Pole, the depletions here are much smaller in size.
Ozone creation and destruction
- The production of ozone in the stratosphere results primarily from the breaking of the chemical bonds within oxygen molecules (O2) by high-energy solar photons. This process, called photodissociation, results in the release of single oxygen atoms, which later join with intact oxygen molecules to form ozone.
- The amount of ozone in the stratosphere varies naturally throughout the year as a result of chemical processes that create and destroy ozone molecules and as a result of winds and other transport processes that move ozone molecules around the planet.
- Over the course of several decades, however, human activities substantially altered the ozone layer.
- Ozone depletion, the global decrease in stratospheric ozone observed since the 1970s, is most pronounced in polar regions, and it is well correlated with the increase of chlorine and bromine in the stratosphere.
- Those chemicals, once freed by UV radiation from the chlorofluorocarbons (CFCs) and other halocarbons (carbon-halogen compounds) that contain them, destroy ozone by stripping away single oxygen atoms from ozone molecules.
- As the amount of stratospheric ozone declines, more UV radiation reaches Earth’s surface, and scientists worry that such increases could have significant effects on ecosystems and human health.
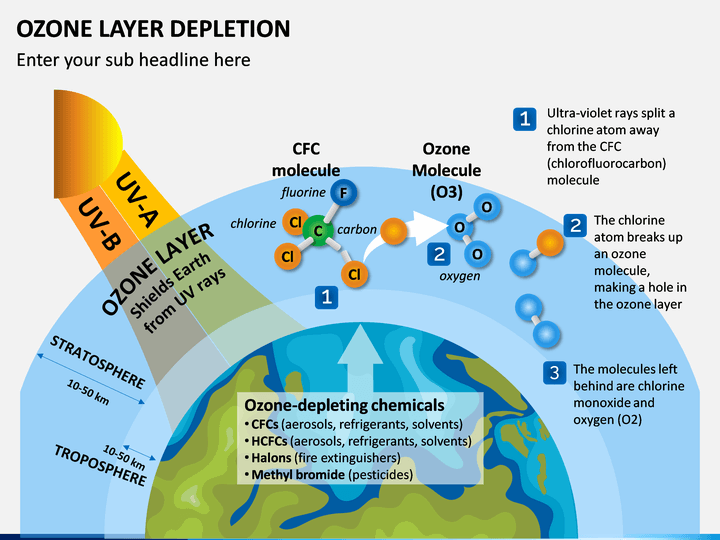
Agreements and steps to address the concerns
- The concern over exposure to biologically harmful levels of UV radiation has been the main driver of the creation of international treaties such as the Montreal Protocol on Substances That Deplete the Ozone Layer and its amendments, designed to protect Earth’s ozone layer. The Montreal Protocol has been a success, with some 99 percent of the ozone-depleting chemicals regulated by the treaty having been phased out since its adoption in 1987. Compliance with international treaties that phased out the production and delivery of many ozone-depleting chemicals, combined with upper stratospheric cooling due to increased carbon dioxide, is thought to have contributed to the shrinking of the ozone holes over the poles and to slightly higher stratospheric ozone levels overall. Continued reductions in chlorine loading are expected to result in smaller ozone holes above Antarctica after 2040
About China’s Ozone-destroying greenhouse gas emissions
- Chlorocarbon, dichloromethane (CH2Cl2) emissions increased in China from 2011-2019, with an average annual increase of 13 per cent primarily from eastern China.
- If global dichloromethane emissions remain at 2019 levels, they could lead to a delay of around five years in Antarctic ozone recovery compared to a scenario with no dichloromethane emissions.
- China accounted for 30-35 per cent of global dichloromethane emissions in 2011-2012. After 2012, emissions from China accounted for 50–60 per cent of the global total.
- The eastern part of China, including part of the North China Plain and the Yangtze River Delta region, are shown to be the main source regions for dichloromethane over the study period.
- The Yangtze River Delta region, which consists of the highly populated provinces Zhejiang, Jiangsu, Shanghai and Anhui, was one of the biggest emitters.
- The increase in emissions from China plays an important role in the global emissions growth, and these increases have the potential to impact the recovery of the stratospheric ozone layer. If global dichloromethane emissions remain at 2019 levels, they could lead to a delay of around five years in Antarctic ozone recovery compared to a scenario with no dichloromethane emissions.
-Source: Down to Earth Magazine




